Interspinous Devices
“CentLoc-LP Spinal Fixation System”
(Obtained approval of the Ministry of Food and Drug Safety and the U.S. FDA.)
This product treats pain or instability caused by damages from fractures and failure of primary surgery resulting from various diseases,incidentsand pathological causes, such as acute and chronic spinal disease, degeneration, deformity, tumor, and stenosis.It is a rear or rear-lateral fixing device. Its components include screws (mono axial screw, poly axial screw, reduction screw, Iliac screw, SAI cannulated screw, SAI non-cannulated screw, and set screw), rod, various connectors, and cross-link, and is an interspinous device that fixes the spine, including the thoracic, lumbar, and sacral vertebrae
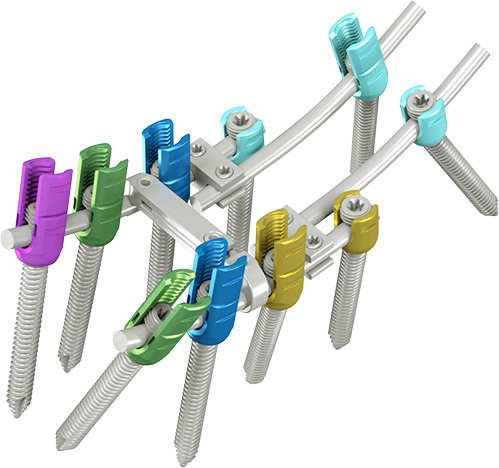
Features
Low profile, top-loading system.
This low-profile system features an enhanced closure mechanism technology and an ergonomically designed instrumentation set.
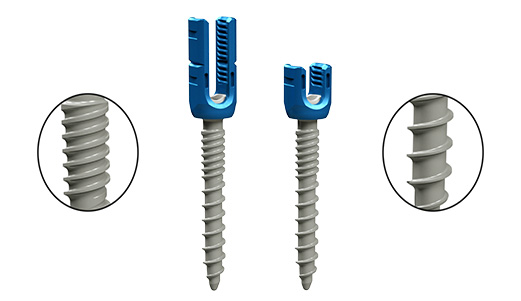
The screws are composed in a way that can be used invarious ways.
The system consists of titanium alloy Poly Axial Screws, Reduction Screws, and Mono Axial Screws in various sizes with high degree of versatility.
Dual-Thread
The screw thread has a dual pitch type, and the spacing of the upper screw thread and the spacing of the lower screw thread are different. The reason is because the nature of the bone area to which eachscrew thread is fixed is different.
- It is designed for quick and easy insertion through dual-thread suitable for cortical bone and cancellous bone.
RootLoc Specialty screw system is designed for the surgeon who is searching the best solution to the spinal fusion for deformity, revision surgery. Lateral Connector, Iliac Screw and side click domino can be used for various environment of the patients.
This product is a ‘medical device’, so please read the ‘Precautions for Use’ and ‘How to Use’ carefully before use.


