Core Competitiveness


FDA-approved Technology
Development of CentLoc, an artificial knee joint that combines biomechanical and ergonomic technologies
Obtained FDA approval
Quality stability has been confirmed by the Ministry of Food and Drug Safety.
-
Key Technology 1

Biomechanical
technology -
Key Technology 2

Ergonomic
modeling technology -
Key Technology 3

Precision casting and
precision machining technology -
Key Technology 4

Surface polishing
treatment technology
- Research Capabilities

Development team andadvisory group of domestic and overseas professional doctors
Product development is carried out in consultation with professional doctors in order to reflect market needs.
Clinical validation of new products is done at affiliated hospitals.
Shareholders and developers

Corporate research center
The Director of the Corporate Research Centeris a former national university professor with field experience (Ph.D. in Materials Science and Engineering).
The Center is led by researchers with 20 to 30 years of field experience in related fields.
We have established a collaboration system for immediately reflecting market needs through consultationwith domestic and overseas advisory groups.

Industry-academiacooperation
Joint product development
Utilization of testing equipment
Joint performance of government projects
- 3D Simulation program design
Ergonomic 3D design by top domestic and overseas medical staff and national university professors
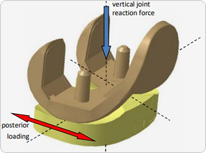

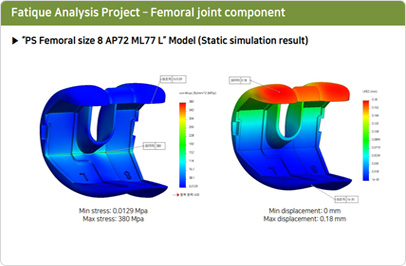


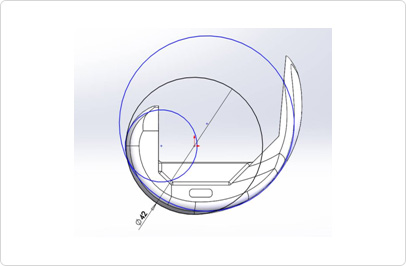


Mechanical Performance Validation Test
- Differentiated Process
We have securedcompetitive advantage in productivity, cost reduction, and quality through the establishment of a batch process line.-

Precision processing
-

Grinding
-

Inspection
-

Sterilization, cleaning, and packaging
- Customer Network
High customer loyalty through cooperation with multiple hospitals participating as product developers
Easy entry into overseas markets utilizing overseas affiliated companies and overseas advisory group networks
-
University hospitals
Major university hospitals
(Improved product
awareness)
-
Special hospitals
Domestic special hospitals
(Sales expansion)
-
Distributors
National distributors
(Establishment of a
national sales network)
-
Overseas markets
Hold US-affiliated companies
Hospitals of overseas
development participants
Established networks in 5 countries
- Differentiated Customer Service
In addition to maintaining high product quality, orthopedic devices such asartificial joints require various instruments needed for surgery.
We seek to differentiate customer services by acquiringmanufacturing technology, producing products, and providing them to surgical sites.
Convenience: Designs that maximize user convenience
Diversity: Various options available
Promptness: Immediate provision to domestic surgery sites through inventory management
Variability: Immediate modification and production of products to suit the needs of each surgical site through domestic manufacturing






